Wright Or MicroPort Profemur Hip Implant Lawsuit
Lawsuit Overview
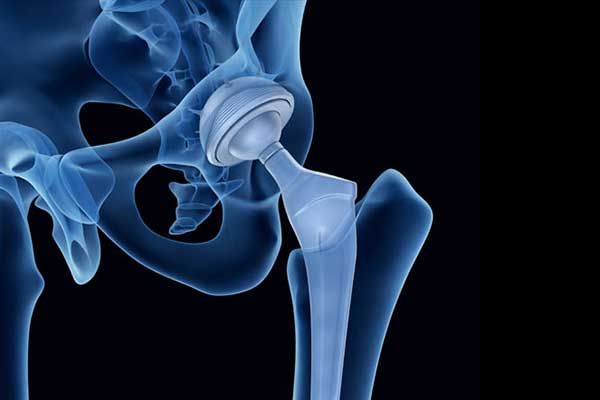
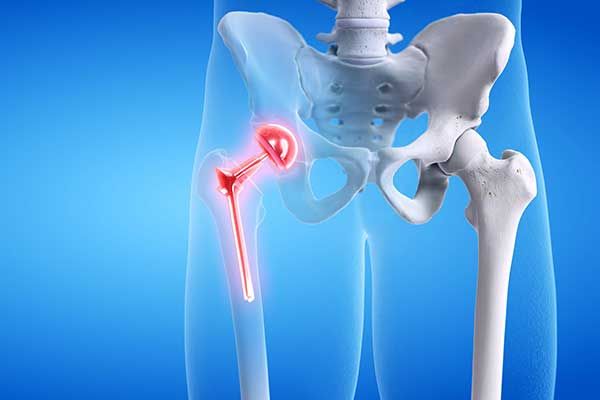
First approved by the FDA in December 2000, the Profemur Modular Hip System is a hip implant designed with a modular neck and stem made of titanium alloy and cobalt chrome. Intended to be a more durable alternative to ceramic or plastic components, the metal-on-metal (MoM) design proved to be the opposite. According to the FDA, MoM hip implants pose risks beyond those of many other artificial hip implants.
Specifically, the Profemur implant is susceptible to unreasonable risk of sudden device fracture, requiring an urgent major surgery to remove the device. The Profemur implant also carries additional risks of other serious injuries, such as:
- Corrosion
- Metallosis, and tissue damage
- Pseudotumor
- Adverse local tissue reaction
- Elevated metals released into a patient’s body
Hundreds of patients have reported Profemur device failures. On December 1, 2008, a “Safety Alert” was sent to certain “medical professionals” notifying of device component failures.
In late 2009, Wright Medical began manufacturing a cobalt chrome version of the titanium modular neck. This was allegedly created to address concerns of:
- Modular neck fractures
- Fretting
- Corrosion
Yet, the cobalt chrome modular necks presented the same problems for patients. This lead to a worldwide recall of the Profemur PHAC 1254 cobalt chrome modular neck. In the most serious type of recall categorized by the FDA, ProFemur Long Cobalt Chrome 8 Degree Varus and Valgus Modular Neck hip implants have been pulled from the market. All 10,825 units were recalled. Many patients who received one of these hip implants have experienced serious injuries requiring surgery to remove and replace the device.
MicroPort, an orthopedic products manufacturer, acquired Wright’s Ortho Recon business in January 2014. Since then MicroPort has made and sold the ProFemur products.
Now, patients are filing lawsuits against the manufacturer with negligence, design defect, manufacturing defect, and failure to warn claims. Plaintiffs have sustained damages and injuries including, but not limited to:
- pain, suffering, and anguish associated with device failure and replacement surgery
- medical bills associated with the replacement procedure, recovery and rehabilitation from the injury
- future medical expenses
- loss of enjoyment of life; and
- loss of earnings capacity
What Is the Problem with the Profemur Hip Implant?
The Profemur Hip Implant carries increased risks for device failure, as well as many other injuries. Susceptible to corrosion, fretting or deteriorating, and complete breakage, the Profemur Hip Implant has shown higher than anticipated rates of failure. Failures in the form of fractures of the modular neck have increased over time.
Patients have experienced adverse side-effects from the device, including:
- Device fracture
- Device breakage
- Metallosis
- Elevated levels of cobalt and/or chromium in the blood
- Corrosion of the device
- Pseudotumor or other adverse local tissue reaction
- Surgical device removal/revision surgery
What is a Metal-on-Metal Implant?
Hip replacement systems made of metal are known as metal-on-metal (MoM) implants. These types of implants have unique risks beyond those of other artificial hip implants. Daily movement inside the body often causes the MoM implant to shed tiny metal particles. This may damage the soft tissue and bone surrounding the implant, and sometimes enter the bloodstream, resulting in a condition called metallosis.
Metallosis is a type of blood poisoning where toxic levels of metal are detected in the bloodstream. Individuals with metallosis—and particularly those with metal-on metal hip implants such as the Profemur Modular Hip System—may be diagnosed with elevated blood levels of cobalt and/or chromium.
MoM implants are also prone to premature fracturing during normal use, causing extreme pain to the patient. Device fracturing, and therefore failure, often requires painful revision surgery to remove or replace the implant.
Who Is Eligible to File a Lawsuit?
You may be legally entitled to compensation if you received a Profemur Hip Implant manufactured by Wright Medical Technology or Microport Orthopedics and experienced adverse side-effects. It’s not too late to investigate your claim.