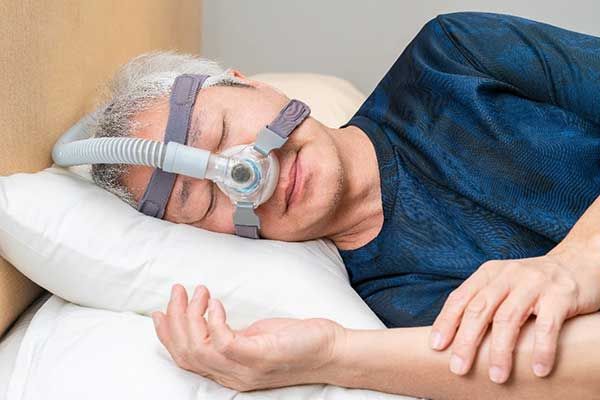
More Concerns Over Philips CPAP Machines
After a June 2021 recall of millions of Philips CPAP and BiPAP breathing machines, consumers are left frustrated, angry, and without answers. The affected machines are equipped with sound abatement foam inside the device which, over time, may degrade into toxic particles. This may lead to other health conditions such as cancer and respiratory conditions.
Philips has explored using an alternative silicone-based foam. However, NBC 10 Boston reports that the FDA said a machine using the replacement foam failed a safety test. The federal safety regulator has ordered Philips to conduct independent testing of the replacement foam. As Philips works to replace machines containing the defective foam, patients may be waiting several months for new devices, leaving them worried about developing other health risks.
Do You Have a Valid Philips CPAP or Bi-PAP Claim?
If you or a loved one used any of the following Philips PAP models for at least one year and developed health conditions such as cancer and/or serious respiratory problems, call our law firm for a free consultation:
- DreamStation CPAP, Auto CPAP, and BiPAP
- DreamStation Go CPAP and APAP
- Dorma 400 and 500 CPAP
- REMStar SE Auto CPAP