Hopeful Couples’ Dreams of Having a Family Dashed by CooperSurgical’s Defective Growth Media
Couples across the country are working through their grief after losing IVF embryos grown in defective CooperSurgical embryo culture media. Lawsuits have been filed against the company to hold them accountable for their negligence in failing to conduct proper testing of the faulty solution—almost 1,000 bottles of which had been distributed worldwide.
For many couples longing to become parents, IVF has been a stressful but rewarding journey. For others, however, it has resulted in heartbreak and devastation.
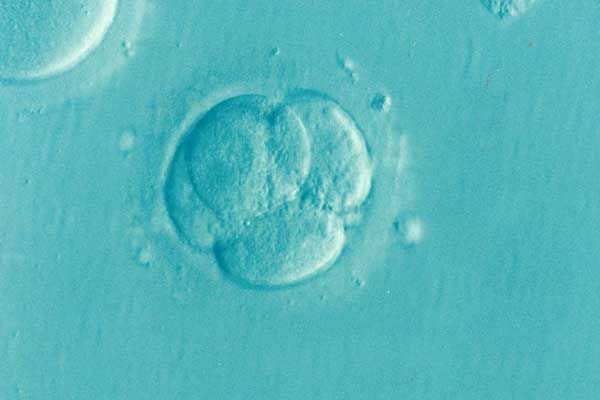
IVF isn’t guaranteed to be successful—yet when the very product meant to support embryo growth is to blame for their failure, it can compound the grief of such a delicate loss. For a couple in Virginia, they experienced this loss firsthand after being informed that their embryos, cultivated in a solution made by CooperSurgical, did not survive.
Their sorrows are unfortunately joined by many other couples who endured similar losses at the same fertility clinic. They were told that the culture solution used to facilitate embryonic growth had been recalled in November and December 2023—the recall had been announced after their precious embryos came in contact with the defective solution.
While these families are still reeling from their losses, many are pursuing legal action against the medical supply company. A couple in Texas has recently sued CooperSurgical after 19 of their embryos were destroyed by the defective culture media. Lawsuits are being filed by plaintiffs who claim that CooperSurgical failed to conduct proper testing of the embryo culture media, and that the defective product along with CooperSurgical’s negligence are to blame for their losses. Some of the lawsuits contend that the defective culture media was missing magnesium, a key ingredient for embryonic growth.
The recall of the LifeGlobal global® Media IVF solution affected three lots distributed to fertility clinics—lot numbers 231020-018741, 231020-018742, and 231020-018743—in more than 30 states and about 25 countries around the world, according to the FDA’s global recall notice posted in February 2024. Nearly 1,000 bottles of the culture media were affected, and almost half of those bottles had been distributed within the United States. Potentially thousands of patients may have been impacted by the defective solution.
Did Your Embryos Fail to Develop in CooperSurgical’s Recalled Culture Media?
If you or a loved one lost an embryo cultivated in CooperSurgical’s recalled defective embryo solution, you may have a claim. Grant & Eisenhofer’s mass tort attorneys Beth Graham, Sindhu Daniel, and Samantha Mertz are experienced in litigation involving women’s health—and specifically reproductive issues—having represented thousands of women in litigation the Essure contraceptive device, power morcellators, and talcum powder. Contact us today to learn about your rights and what potential recoveries may be available to you to pursue due to this recall. We are a team of dedicated attorneys here for you during this difficult time.